I. The Human Dream of Defying Time
Since the dawn of civilization, humans have been captivated by the idea of transcending mortality. Ancient myths across cultures—whether the Sumerian Epic of Gilgamesh, the Chinese tales of the elixir of immortality, or the Greek legends of ambrosia—reflect a timeless aspiration: to extend life beyond its natural limits. For centuries, this ambition was confined to poetry, religion, and alchemy.
The scientific pursuit of longevity began only in the last two centuries, when biology shifted from speculative philosophy to experimental science. Today, advances in gene editing have brought humanity closer than ever to reshaping the very biological mechanisms of aging. To understand this journey, we must first trace the historical roots of genetic science.
II. From Mendel’s Peas to the Blueprint of Life
1. The Birth of Genetics
In the mid-19th century, an Austrian monk named Gregor Mendel observed patterns of inheritance in pea plants. His discovery of discrete hereditary “factors,” later named genes, laid the foundation for modern genetics. Although Mendel’s work was largely ignored during his lifetime, it was rediscovered at the turn of the 20th century, inspiring a new generation of biologists.
2. The Double Helix and the Molecular Era
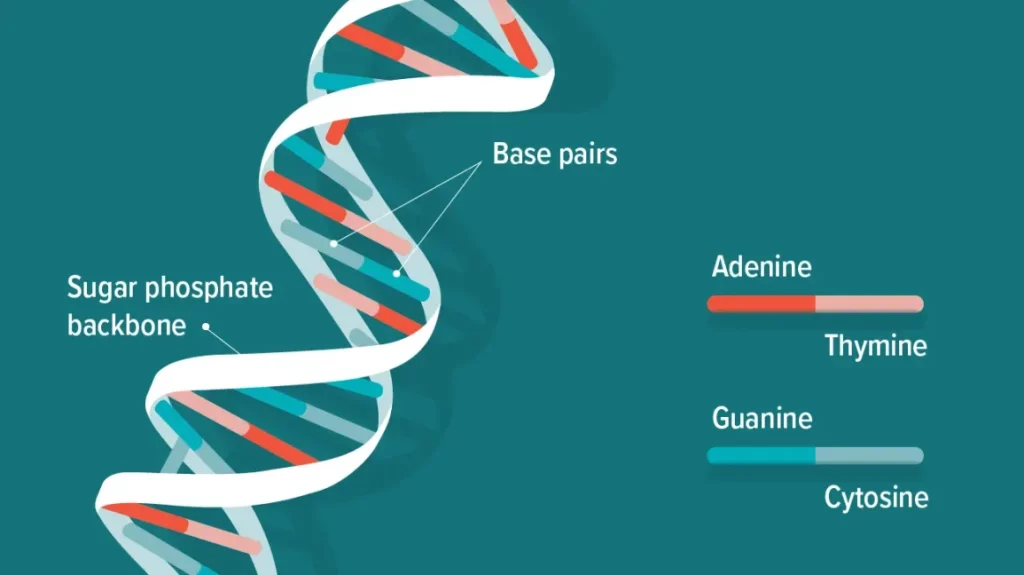
A major leap came in 1953 when James Watson and Francis Crick, building on Rosalind Franklin’s X-ray crystallography data, unveiled the double-helix structure of DNA. This revelation turned biology into an information science. DNA was now seen as a universal code—a sequence of nucleotides carrying instructions for every protein and process in living organisms.
3. The Age of Genomics
The discovery of DNA’s structure sparked decades of research into decoding this genetic language. Milestones such as the polymerase chain reaction (PCR) in the 1980s and the Human Genome Project (1990–2003) revolutionized our understanding of life’s blueprint. By mapping all 3 billion base pairs in human DNA, scientists gained an unprecedented view of the genetic factors influencing disease and aging.
III. Aging as a Biological Process
For most of history, aging was considered an inevitable, mysterious decline. Modern biology reframes it as a complex, multifactorial process governed by genetic and epigenetic mechanisms.
1. The Telomere Clock
In the 1970s, researchers discovered that chromosomes have protective caps called telomeres. With each cell division, these telomeres shorten, eventually leading to cellular senescence—a state where cells stop dividing and lose functionality. Elizabeth Blackburn, Carol Greider, and Jack Szostak, awarded the 2009 Nobel Prize, identified the enzyme telomerase, which can replenish telomeres and delay cellular aging.
2. Genetic Pathways of Longevity
Studies in model organisms such as yeast, worms (C. elegans), and mice revealed genes like FOXO3, SIRT1, and IGF-1 that influence lifespan by modulating metabolism, stress resistance, and DNA repair. Caloric restriction experiments further demonstrated that altering cellular pathways could significantly extend lifespan.
3. Epigenetic Drift
Beyond the genome, aging is shaped by epigenetic changes—chemical modifications that regulate gene expression without altering DNA sequences. Over time, epigenetic “marks” drift, leading to dysregulated gene activity. This discovery suggests that aging is not just genetic fate but also potentially reversible.
IV. The Gene-Editing Revolution
1. Early Tools: Zinc Fingers and TALENs
Before CRISPR, scientists developed targeted DNA modification tools such as zinc-finger nucleases and TALENs. While powerful, these technologies were costly, labor-intensive, and limited in scope.
2. CRISPR-Cas9: A Scientific Breakthrough
In 2012, Jennifer Doudna and Emmanuelle Charpentier unveiled CRISPR-Cas9, adapted from a bacterial immune system. CRISPR acts like molecular scissors guided by RNA, enabling precise and efficient editing of almost any DNA sequence. This democratized gene editing, transforming it from a specialist’s art into a widely accessible tool.
3. Beyond CRISPR: Prime Editing and Base Editing
Recent innovations such as base editors and prime editing offer even greater precision, minimizing unintended mutations. These advances open the door to safely correcting age-related genetic defects or enhancing protective genes.
V. Gene Editing Meets the Biology of Aging
1. Senescent Cell Clearance
Aging tissues often accumulate senescent cells that secrete inflammatory molecules, contributing to age-related diseases. Experimental therapies combine CRISPR-based targeting with senolytic drugs to selectively eliminate these harmful cells.
2. Telomere Restoration
Gene therapies delivering telomerase have extended lifespan in animal models without triggering cancer, which remains a critical safety concern. Controlled activation of telomerase could rejuvenate tissues and delay age-related decline.
3. Mitochondrial Rejuvenation
Mitochondria, the cell’s energy factories, deteriorate with age due to accumulated mutations. Techniques like mitochondrial gene transfer and CRISPR-based repair aim to restore energy production, potentially reversing age-related degeneration.
4. Epigenetic Reprogramming
Recent experiments by Shinya Yamanaka’s team showed that partially reprogramming adult cells with specific transcription factors can reset their epigenetic clocks, restoring youthful function. This discovery hints at future therapies to rejuvenate entire organs.
VI. Challenges and Cautions
1. Complexity of Aging
Aging is not driven by a single gene but by interconnected processes. Editing one pathway may yield limited benefits or cause unintended consequences.
2. Cancer Risks
Interventions that promote cell division, such as telomerase activation, can increase cancer susceptibility. Balancing rejuvenation with genomic stability remains a major hurdle.
3. Ethical Boundaries
While treating age-related diseases is widely accepted, editing embryos to enhance longevity raises profound ethical questions about consent, inequality, and potential misuse.
4. Societal Implications
If only the wealthy can afford life-extending therapies, society could face new forms of inequality. Global governance will be essential to ensure equitable access.
VII. The Road Ahead
The dream of extending human lifespan through genetic intervention is no longer confined to science fiction. Clinical trials are underway for therapies targeting age-related blindness, muscular atrophy, and cardiovascular degeneration.
Future breakthroughs will likely emerge at the intersection of gene editing, stem cell therapy, and AI-driven precision medicine. Yet progress must proceed with caution, guided by ethical frameworks and robust public dialogue.
Humanity now stands at the threshold of a profound transformation—one where the genetic code itself becomes a tool to reshape the trajectory of life. Whether this power is used to create a healthier, longer-lived society or exacerbates existing inequalities will depend on the choices we make today.